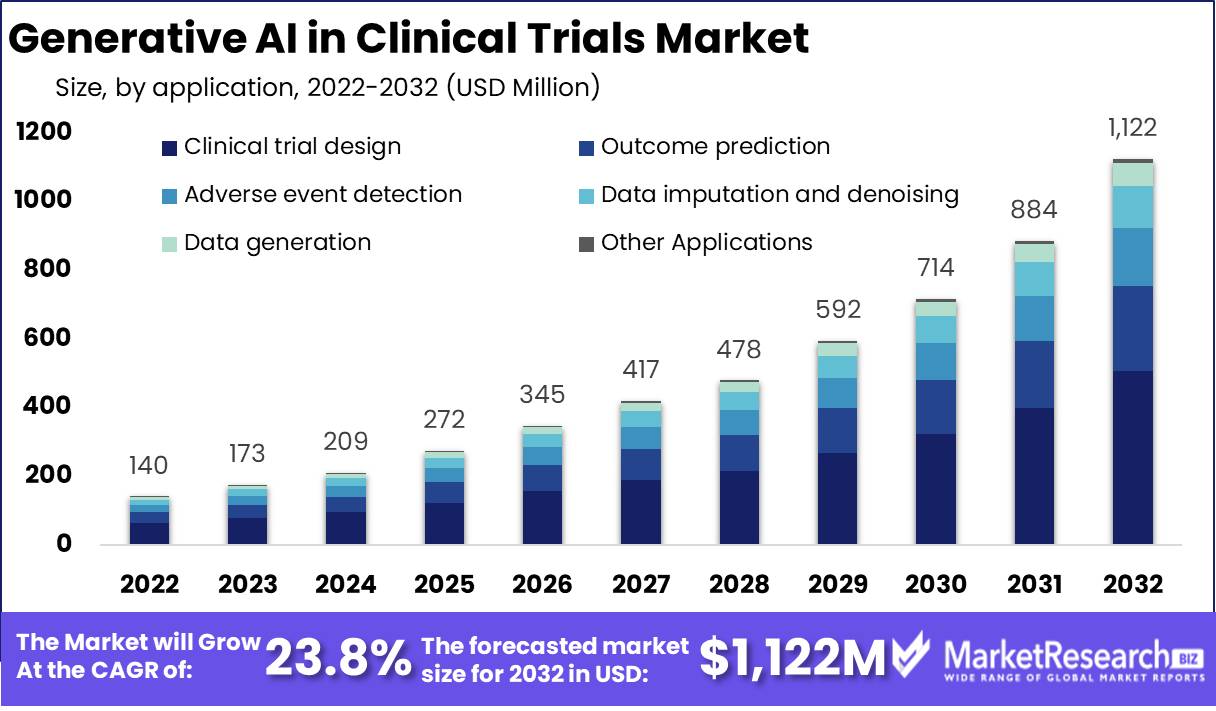
Generative AI in Clinical Trials Market Size is expected to be worth around USD 1,122 Mn by 2032
Page Contents
Market Overview
Published Via 11Press : Generative AI in Clinical Trials Market size is expected to be worth around USD 1,122 Mn by 2032 from USD 140 Mn in 2022, growing at a CAGR of 23.8% during the forecast period from 2022 to 2032.
Generative artificial intelligence (AI) in clinical trials has gained much interest and could transform healthcare research. Generative AI refers to a subset of AI that creates new data rather than simply analyzing existing information; when applied to trials, this technology can streamline various aspects of trial design, patient recruitment, data analysis and monitoring, drawing in pharmaceutical companies, contract research organizations (CROs) and regulatory bodies alike.
Generic AI offers many advantages to clinical trial design. By analyzing large volumes of patient data, AI algorithms can identify patterns and generate hypotheses that researchers may have missed; these insights allow more efficient trial designs with improved patient outcomes at reduced costs. Additionally, this technology enables researchers to simulate virtual patient populations for testing different scenarios or interventions before engaging in expensive and time-consuming clinical research studies.
Generational AI is also making waves in patient recruitment. Patient recruitment has traditionally been one of the major obstacles to clinical trials, often leading to delays and higher costs. By harnessing various data sources – like electronic health records or social media posts – Generative AI enables researchers to automate and speed up recruitment processes for clinical trials more efficiently; helping ensure timely completion.
Generative AI also plays an essential role in data analysis and interpretation. Given the growing volume and complexity of clinical trial data, traditional methods may no longer suffice. Generative AI algorithms are adept at handling large datasets while simultaneously recognizing patterns, relationships, or correlations that would not otherwise be obvious to human researchers; this helps unearth valuable insights, predict patient responses accurately, or identify subpopulations that may benefit from interventions that target them specifically.
Generative AI presents numerous opportunities, but there are some unique challenges associated with its use that must be considered and overcome. Ensuring data privacy and security are paramount when working with sensitive patient data; regulatory bodies must develop guidelines and frameworks to guide the use of generative AI in clinical trials while guaranteeing transparency and ethical considerations are adhered to.
Request Sample Copy of Generative AI in Clinical Trials Market Report at: https://marketresearch.biz/report/generative-ai-in-clinical-trials-market/request-sample
Key Takeaways
- Generative AI could transform healthcare research by producing new data and optimizing study design.
- Artificial intelligence algorithms can analyze patient data and generate hypotheses to produce more efficient and targeted trials.
- Generative AI allows researchers to simulate virtual patient populations and test interventions prior to conducting real studies.
- AI-powered patient recruitment can significantly speed up and streamline enrollment processes, minimizing delays and costs.
- Generative AI algorithms are powerful ways to analyze large datasets and discover valuable insights, providing more effective data analysis and interpretation.
- Data privacy and security should always be of primary concern when conducting clinical trials using generative AI.
- Regulatory guidelines and frameworks must be in place in order to guarantee transparency and ethical use of generative AI in research settings.
- Generative AI offers great potential to accurately anticipate patient responses and pinpoint subpopulations that could benefit from specific interventions.
- Responsible application of generative AI can result in more effective treatment options and enhanced patient outcomes.
Regional Snapshot
North America and especially the US is at the forefront of adopting generative AI in clinical trials, with major pharmaceutical companies, academic institutions, and technology firms investing heavily in research and development for these trials. Furthermore, FDA has shown its support for AI technologies by initiating efforts to streamline regulatory processes for AI-powered trials.
Europe is witnessing significant advances in generative AI for clinical trials. Countries such as Great Britain, Germany and France are making strides to use artificial intelligence (AI) in healthcare research; European Medicines Agency (EMA) has recognized AI's potential and is working on guidelines to ensure its safe and effective usage during trials.
Asia-Pacific countries are seeing rapid progress in adopting artificial intelligence-powered clinical trials. China, Japan and South Korea are actively investing in AI research and development projects with healthcare applications in mind; China alone invests over $550 million per year. With such a large population and diversity among patients present here there are ample opportunities for AI-powered trials as well as data analysis.
Though generative AI in clinical trials remains rare in Latin America, there has been increasing interest in using this technology for clinical research purposes. Brazil and Mexico are emerging as key countries, with academic institutions and research organizations exploring AI for optimizing trial design and patient recruitment purposes.
Middle East and Africa region is increasingly adopting artificial intelligence in clinical trials. Countries like Israel and South Africa have emerged as global leaders for AI research and development, and efforts are currently being undertaken to employ AI algorithms for personalized medicine, drug discovery, and trial optimization purposes.
For any inquiries, Speak to our expert at: https://marketresearch.biz/report/generative-ai-in-clinical-trials-market/#inquiry
Drivers
Enhance Efficiency and Achieve Cost Savings
One of the primary drivers behind adopting generative AI in clinical trials is its capacity to increase efficiency and cut costs. By optimizing study design and patient recruitment processes, generative AI can speed up trial processes and deliver results faster with lower associated expenses – translating to significant cost savings for pharmaceutical companies and research organizations alike.
Increase Patient Recruitment and Retention Capabilities
Patient recruitment is an integral aspect of clinical trials, and generative AI can significantly facilitate this process. AI algorithms can efficiently parse massive volumes of patient data to quickly identify eligible participants – streamlining recruitment procedures while cutting time and effort consumption significantly. Additionally, this type of technology can aid retention efforts by recognizing issues early and creating personalized strategies designed to increase engagement and compliance among participants.
Data Analysis and Insights
As clinical trial data becomes ever more complex and volumetric, traditional analysis methods become increasingly challenging to manage. Generative AI algorithms provide an effective solution by quickly sorting large datasets to identify patterns, correlations, and potential insights missed by human researchers – empowering researchers with the capability to make more informed decisions, predict patient responses accurately, identify subpopulations that would benefit from interventions more effectively, ultimately leading to improved outcomes.
Personalised Medicine and Optimized Therapy Options
Generative AI holds great promise to revolutionize personalized medicine by tailoring treatments specifically to each patient. AI algorithms can analyze patient data to detect biomarkers, genetic variations, or any other factor which might impede treatment responses; using this knowledge they can assist with designing targeted interventions or optimizing treatment plans that result in more effective therapies and enhanced patient care.
Restraints
Data Privacy and Security Concerns
Generative AI's use in clinical trials requires gathering and analyzing sensitive patient data, so ensuring its privacy and security is of utmost importance in order to safeguard patient confidentiality and fulfill regulatory requirements. Risks related to breaches or unauthorized access pose considerable hurdles to the adoption of Generative AI technology in trials.
Regulatory Challenges
The regulatory landscape surrounding AI in healthcare remains complex, creating challenges to its implementation in clinical trials. Regulating bodies must develop guidelines and frameworks to govern ethical and responsible use of generative AI; while also adhering to data protection regulations. Navigating through these regulations and obtaining necessary approvals may take considerable time and resources.
Lack of Standardization
A lack of standardized protocols and methodologies for the implementation of generative AI in clinical trials may impede its widespread adoption. Without clear guidelines to follow, different organizations could differ in how they utilize this form of artificial intelligence; leading to inconsistencies in data analysis and interpretation across studies and institutions. Standardization efforts are crucial in order to guarantee interoperability and reproducibility across studies and institutions.
Integrate Existing Systems and Workflows
Integrating generative AI into existing clinical trial systems and workflows may pose technical hurdles. Integration with electronic health records (EHRs), data management platforms, and existing infrastructure requires careful planning and coordination; compatibility issues between legacy systems and newer generative AI solutions could further hamper implementation processes.
Opportunities
Accelerated Drug Discovery and Development
Generative AI holds the promise to revolutionize drug discovery by helping identify novel targets, and predict efficacy and safety profiles of potential drug candidates. Through simulating virtual experiments and analyzing large datasets, generative AI can quickly identify promising compounds, saving both time and costs associated with drug development.
Real-Time Monitoring and Adaptive Trials
Generative AI allows real-time monitoring of patients during clinical trials, providing insights into treatment responses, adverse events and other data relevant to clinical trial designs. Such real-time monitoring enables adaptive trial designs where trial parameters and interventions can be adjusted according to emerging data trends for enhanced efficiency and success rates in clinical trials.
Collaboration and Data Sharing
Generative AI's use in clinical trials presents new opportunities for collaboration and data sharing between researchers and organizations. AI algorithms can analyze aggregated datasets from multiple trials to allow researchers to draw more comprehensive conclusions, identify rare adverse events and gain insight from diverse patient populations. Collaboration platforms and initiatives facilitate knowledge transfer thereby furthering medical research.
Enhance Patient Engagement and Experience
Generative AI can play an instrumental role in increasing patient engagement and satisfaction during clinical trials. Chatbots powered by AI provide patients with personalized information, reminders and support tailored to them – further increasing involvement and adherance to trial protocols – thus leading to higher retention rates and more reliable study results.
Take a look at the PDF sample of this report: https://marketresearch.biz/report/generative-ai-in-clinical-trials-market/request-sample
Challenges
Considerations on Ethics
Care must be taken when employing generative AI for clinical trials, with its ethical repercussions carefully addressed. Issues surrounding informed consent, data privacy, algorithm bias, transparency in decision-making and transparency need to be carefully considered before deployment occurs in a manner that upholds public trust while upholding integrity in research.
Human-AI Collaboration
Generative AI may identify patterns and correlations in large datasets, yet its findings may only apply to the specific population or context used when training the AI algorithms. Extrapolating results beyond these specific settings may present challenges and require further validation through real-world studies.
Explainability and Interpretability (III).
Generative AI algorithms often operate like black boxes, making their decision-making processes hard to decipher and interpret. Explaining why an AI arrived at a specific recommendation or conclusion may prove challenging in complex clinical scenarios; hence ensuring the interpretability and explainability of outputs generated by these AI systems is vital in garnering trust among healthcare professionals and regulatory authorities.
Human-AI Collaboration
Successful implementation of generative AI into clinical trials demands seamless cooperation between AI systems and human researchers. Building trust and encouraging interdepartmental cooperation is vital; developing workflows and communication channels that leverage both bits of intelligence will also be required to optimize results.
Market Segmentation
Based on Application
- Data generation
- Clinical trial design
- Outcome prediction
- Adverse event detection
- Data imputation and Denoising
- Other
Based on Technology
- Variational Autoencoders (VAEs)
- Generative Adversarial Networks (GANs)
- Deep Convolutional Networks (DCNs)
- Transfer Learning
- Other
Based on End-Use
- Researchers and Scientists
- Healthcare Professionals
- Clinical Trial Sponsors and CROs
- Data Analysts and Biostatisticians
- Other
Key Players
- IBM Watson
- Microsoft Corporation
- Google LLC
- Tencent Holdings Ltd.
- Neuralink Corporation
- Johnson & Johnson
- Other
Report Scope
| Report Attribute | Details |
| Market size value in 2022 | USD 140 Mn |
| Revenue Forecast by 2032 | USD 1,122 Mn |
| Growth Rate | CAGR Of 23.8% |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, and Rest of the World |
| Historical Years | 2017-2022 |
| Base Year | 2022 |
| Estimated Year | 2023 |
| Short-Term Projection Year | 2028 |
| Long-Term Projected Year | 2032 |
Request Customization Of The Report: https://marketresearch.biz/report/generative-ai-in-clinical-trials-market/#request-for-customization
Recent Developments
- In 2022, Insilico Medicine and Pfizer announced a collaboration to jointly research novel small molecules using Insilico Medicine's artificial intelligence platform and identify and optimize potential drug candidates faster; eventually accelerating clinical trials across various therapeutic areas.
- In 2021, BenevolentAI and AstraZeneca joined forces to explore how artificial intelligence (AI) could aid drug discovery and development. BenevolentAI's AI platform uses generative AI algorithms to efficiently search vast amounts of scientific literature and data, in order to identify new targets for therapeutic interventions as well as potential therapies.
- In 2022, Verge Genomics raised $99 Million in Series C funding in order to further advance their AI platform for drug discovery and clinical trials. Their approach integrates genomics, transcriptomics and generative AI in order to quickly identify disease-specific drug targets as well as accelerate novel therapy development.
- In 2023, Exscientia announced a collaboration with Bayer to identify small molecule drug candidates for cardiovascular diseases using AI technology. Through their AI platform, Exscientia utilizes generative AI algorithms to design molecules with desired properties to expedite clinical trial processes for these therapies.
FAQ
1. What are the characteristics of generative AI used in clinical trials?
A. Generative AI refers to the application of artificial intelligence algorithms which can create new data and formulate hypotheses in clinical trials.
2. How does AI improve patient recruitment?
A. Generative AI utilizes patient data from various sources to quickly and efficiently identify eligible trial participants, thus speeding recruitment processes and expediting clinical trial completion on time.
3. What are the advantages of employing generative AI for clinical trials?
A. Generative AI offers several benefits to its users, such as optimized study design, cost reductions, increased patient engagement and uncovering hidden insights in complex datasets.
4. What challenges does Generative AI present during clinical trials?
A. Challenges associated with data privacy and security include regulatory compliance, algorithm bias mitigation, standardization, and integration into existing systems and workflows.
5. How does Generative AI assist with data analysis and interpretation?
A. Generative AI algorithms can analyze large datasets quickly and efficiently, quickly recognizing patterns, correlations, and potential relationships that humans might miss, leading to more accurate data analysis and interpretation.
6. What ethical considerations exist in using generative AI for clinical trials?
A. Ethical considerations involve obtaining informed consent, safeguarding data privacy and privacy laws, addressing algorithm bias, adhering to regulatory guidelines, and adhering to best practices when using generative AI technologies responsibly and transparently.
7. Can generative AI accelerate drug discovery for clinical trials?
A. Yes, generative AI can expedite drug discovery by quickly identifying novel targets, optimizing molecules and predicting efficacy and safety profiles of potential drug candidates – saving both time and costs in the process.
Contact us
Contact Person: Mr. Lawrence John
Marketresearch.Biz
Tel: +1 (347) 796-4335
Send Email: [email protected]
Content has been published via 11press. for more details please contact at [email protected]
The team behind market.us, marketresearch.biz, market.biz and more. Our purpose is to keep our customers ahead of the game with regard to the markets. They may fluctuate up or down, but we will help you to stay ahead of the curve in these market fluctuations. Our consistent growth and ability to deliver in-depth analyses and market insight has engaged genuine market players. They have faith in us to offer the data and information they require to make balanced and decisive marketing decisions.