Neuroendoscopy Devices Market to Cross USD 326 Mn in 2033 | 5.30% CAGR
Page Contents
Neuroendoscopy Devices Market Overview
Published Via 11Press: Neuroendoscopy devices are minimally invasive surgical instruments used to treat neurological disorders such as hydrocephalus, brain tumors, and intracranial hemorrhage. Through small incisions in the skull, surgeons can access the brain with minimal damage to surrounding tissues.
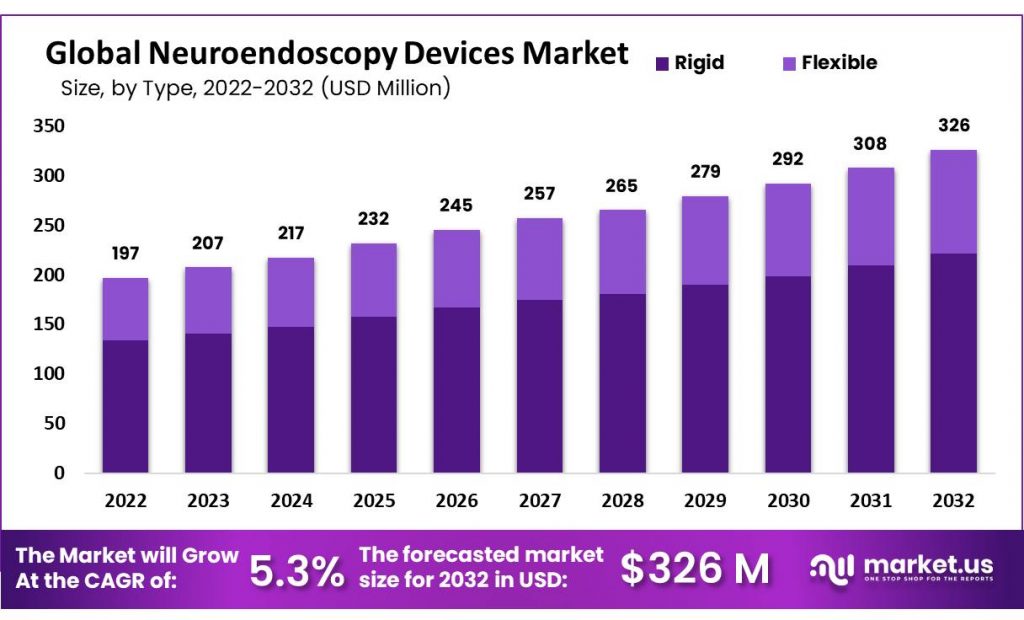
The global neuroendoscopy devices market size is forecast to reach USD 326 million by 2032 from USD 197 million in 2022, rising at a compound annual growth rate (CAGR) of 5.30% during the forecast period.
From 2022 to 2032, neuroendoscopy devices come in several varieties such as rigid endoscopes, flexible endoscopes and semi-rigid endoscopes. Rigid endoscopes are the most widely used type of endoscopy.
Neuroendoscopes are the preferred surgical view, offering a clear and stable view of the surgical site. However, flexible endoscopes have become increasingly popular due to their ability to navigate through intricate medical conditions.
The market is further segmented based on application, with the major applications including intraventricular endoscopy, transnasal endoscopy, and transcranial endoscopy. Intraventricular endoscopy accounts for 40% of the market share while transnasal and transcranial endoscopy account for 36% each.
Geographically, North America leads the market for neuroendoscopy devices, followed by Europe and Asia-Pacific. The rising prevalence of neurological disorders and increasing adoption of minimally invasive surgeries in these regions are driving the market growth.
Some of the key players in the neuroendoscopy devices market include Ackermann Instrument GmbH, B Braun Meslsungen AG, HAWK, Clarus Medical LLC, KARLSTORZSE & CO. AG, Adeor Medical AG, Nevro Corporation, Tonglu WANHE Medical Instrument, Machida Endoscope, Schindler endoscopy Technology GmbH, Olympus Corporation, Boston Scientific Corporation, Aesculap, Inc., KARL STORZ AE & CO, Medical Instruments Co, Ltd., Evonos. These companies are focusing on developing new and advanced neuroendoscopy devices to stay competitive in the market.
Key Takeaways
- The global neuroendoscopy devices market is expected to grow at a CAGR of around 5.30% from 2022 to 2032.
- The increasing incidence of neurological disorders, technological advancements in neuroendoscopy devices, and the growing demand for minimally invasive surgeries are driving the market growth.
- Rigid endoscopes are the most commonly used type of neuroendoscopy device, followed by flexible and semi-rigid endoscopes.
- The major applications of neuroendoscopy devices are intraventricular endoscopy, transnasal endoscopy, and transcranial endoscopy.
- North America is the largest market for neuroendoscopy devices, followed by Europe and Asia-Pacific.
- Key players in the neuroendoscopy devices market include Ackermann Instrument GmbH, B Braun Meslsungen AG, HAWK, Clarus Medical LLC, KARLSTORZSE & CO. AG, Adeor Medical AG, Nevro Corporation, Tonglu WANHE Medical Instrument, Machida Endoscope, Schindler endoscopy Technology GmbH, Olympus Corporation, Boston Scientific Corporation, Aesculap, Inc., KARL STORZ AE & CO, Medical Instruments Co, Ltd., Evonos.
- These companies are focusing on developing new and advanced neuroendoscopy devices to stay competitive in the market.
Request For Sample Report: https://market.us/report/neuroendoscopy-devices-market/request-sample/
Regional Snapshot
- North America: The neuroendoscopy market is the largest in North America, driven by the high prevalence of neurological disorders and the growing demand for minimally invasive surgeries. The United States accounts for the majority of this growth in this region.
- Europe: The neuroendoscopy market is the second-largest in Europe, driven by an increase in minimally invasive surgeries and access to advanced healthcare infrastructure.Allemagne, France, and the United Kingdom are major contributors to growth within this region.
- Asia-Pacific: This is the fastest-growing market for neuroendoscopy devices, driven by an increasing prevalence of neurological disorders, an aging geriatric population, and rising healthcare spending. China, Japan, and India are the major contributors to this region's growth in the healthcare facilities market.
- Latin America: A small but growing market for neuroendoscopy devices has emerged due to the rising adoption of minimally invasive surgeries and expanding healthcare infrastructure within this region. Brazil and Mexico are leading contributors to this growth.
- Middle East & Africa: Neuroendoscopy devices are seeing a small but growing market due to the rising prevalence of neurological disorders and demand for advanced healthcare facilities. South Africa and Saudi Arabia are two major contributors to this growth in this region.
Drivers
- Increased Incidence of Neurological Disorders: With the rising incidence of neurological conditions like brain tumors, hydrocephalus, and intracranial hemorrhage, the demand for neuropsychological services is growing.
- Technological Advancements: The introduction of advanced neuroendoscopy devices with improved visualization and maneuverability is fueling market expansion.
- Growing Demand for Miniature-Invasive Surgery: The growing preference for minimally invasive surgeries is encouraging the adoption of neuroendoscopy devices, which provide less invasive procedures with fewer complications and faster recoveries.
- Increased Healthcare Expenditure: The rising healthcare expenditure across both developed and developing countries is fueling demand for advanced medical devices such as neuroendoscopy.
- Aging Population: With an aging population comes an increased vulnerability to neurological disorders, leading to the growing demand for neuroendoscopy devices.
- Favorable reimbursement policies: The availability of favorable reimbursement policies for neuroendoscopy procedures is fueling market growth by making these procedures more accessible and affordable to patients.
Restraints
- High cost of neuroendoscopy procedures: Neuroendoscopy procedures can be expensive, which may limit their adoption in certain regions or by certain patient populations.
- Limited availability of skilled healthcare professionals: Neuroendoscopy procedures require highly skilled healthcare professionals, and there may be a limited number of trained professionals in certain regions.
- Risks associated with neuroendoscopy procedures: While minimally invasive, neuroendoscopy procedures still carry some risks such as bleeding, infection, and damage to surrounding tissues. This may deter some patients from undergoing the procedure.
- Stringent regulatory requirements: The regulatory requirements for medical devices can be stringent, which may delay the approval and commercialization of new neuroendoscopy devices.
- Lack of awareness and infrastructure in developing countries: The lack of awareness about neuroendoscopy procedures and the limited healthcare infrastructure in some developing countries may limit market growth in these regions.
Opportunities
- Emerging Markets: There is a significant growth potential in emerging markets such as Asia-Pacific and Latin America, where there is an increasing demand for advanced medical devices and an expanding healthcare infrastructure.
- Development of Advance Neuroendoscopy Devices: The introduction of cutting-edge neuroendoscopy devices with enhanced visualization, maneuverability and safety capabilities presents an immense market growth potential.
- Increased Demand for Outpatient Procedures: The trend towards outpatient procedures is on the rise, providing neuroendoscopic procedures with an opportunity to be performed outside of the hospital setting.
- Technological Advancements in Imaging: Recent advances in imaging technologies like MRI and CT scans are improving preoperative diagnosis and planning, which could potentially increase surgical success rates.
- Telemedicine and Remote Surgery: Advances in telemedicine and remote surgery technologies could make neuroendoscopy procedures more accessible, potentially cutting down on costs as well.
- Collaborations and Partnerships: Partnerships between industry players and healthcare providers could increase awareness about neuroendoscopy procedures and spur market expansion.
Challenges
- Stringent Regulatory Requirements: Medical device regulations can be rigorous, potentially delaying approval and commercialization of new neuroendoscopy devices.
- Competition from Alternative Treatment Options: Neuroendoscopy procedures face competition from alternative treatments such as traditional open surgery, radiation therapy, and more.
- High Cost of Neuroendoscopy Procedures: Neuroendoscopy procedures may be costly, which may limit their availability in certain regions or among certain patient populations.
- Lack of Skilled Healthcare Professionals: Neuroendoscopy procedures require highly experienced healthcare professionals, and in certain areas there may be a shortage of trained personnel.
- Limited reimbursement policies: Limited or no reimbursement policies for neuroendoscopy procedures may prevent their adoption, particularly in regions with high patient volumes.
- Neuroendoscopy procedures carry risks: Though minimally invasive, neuroendoscopy still poses potential risks such as bleeding, infection and tissue damage. Therefore, some patients may choose not to undergo the procedure due to potential risks.
Grow your profit margin with Market.us Get this Report
Recent Developments
- In 2021, Stryker Corporation launched its Neuroform Atlas Stent System, which is designed for use in treating unruptured wide-necked aneurysms.
- In 2020, Karl Storz SE & Co. KG launched its new KINEVO 900 surgical microscope, which features 4K resolution and augmented reality capabilities, allowing for more precise neuroendoscopy procedures.
- In 2019, Medtronic plc received FDA approval for its Stealth Autoguide Cranial Software, which is designed to assist with the placement of cranial implants during neuroendoscopy procedures.
- In 2019, Olympus Corporation launched its EndoTherapy Disposables portfolio, which includes a range of accessories and devices for use in neuroendoscopy procedures.
- In 2018, B. Braun Melsungen AG introduced its Neuro 3D Navigation System, which features real-time tracking and visualization capabilities for use in neuroendoscopy procedures.
- In 2018, Integra LifeSciences Corporation acquired the neurosurgery assets of Arkis BioSciences, including its CerebroFlo external drainage and monitoring system for use in neuroendoscopy procedures.
Key Market Segments
Based on Type
- Rigid
- Flexible
Based on Surgery
- Intraventricular
- Transnasal
- Transcranial
Based on Usability
- Reusable
- Disposable
Based on End-User
- Hospitals
- Diagnostic Centre’s
- Ambulatory Surgical Centers
Market Key Players
- Ackermann Instrument GmbH
- B Braun Meslsungen AG
- HAWK
- Clarus Medical LLC
- KARLSTORZSE & CO. AG
- Adeor Medical AG
- Nevro Corporation
- Tonglu WANHE Medical Instrument
- Machida Endoscope
- Schindler endoskopie Technology GmbH
- Olympus Corporation
- Boston Scientific Corporation
- Aesculap, Inc.
- KARL STORZ AE & CO
- Medical Instruments Co, Ltd.
- Evonos
Nature Insights
The Neuroendoscopy Devices market is a fiercely competitive and rapidly developing arena that's driven by technological innovations and advancements.
The rising prevalence of neurological disorders like brain tumors, stroke, and hydrocephalus as well as an increasing need for minimally invasive surgical procedures is all driving this trend.
Furthermore, the market is witnessing a trend toward advanced neuroendoscopy devices with enhanced visualization, maneuverability, and safety features. This trend bodes well for future growth prospects in this space.
The adoption of telemedicine and remote surgery technologies is predicted to accelerate market expansion. Furthermore, they will increase accessibility for neuroendoscopy procedures while decreasing costs significantly.
Report Scope
| Report Attribute | Details |
| The market size value in 2022 | USD 197.0 Mn |
| Revenue forecast by 2032 | USD 326.0 Mn |
| Growth Rate | CAGR Of 5.3% |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, and the Rest of the World |
| Historical Years | 2017-2022 |
| Base Year | 2022 |
| Estimated Year | 2023 |
| Short-Term Projection Year | 2028 |
| Long-Term Projected Year | 2032 |
FAQ.
Neuroendoscopy devices are minimally invasive surgical instruments used in neurosurgery to visualize and treat neurological conditions such as brain tumors, stroke, and hydrocephalus.
The neuroendoscopy devices market is primarily being driven by an increasing prevalence of neurological disorders, the rising demand for minimally invasive surgical procedures, and technological advances.
The neuroendoscopy devices industry faces numerous difficulties, including stringent regulatory requirements, competition from alternative treatment options, high costs, lack of skilled healthcare professionals, and limited reimbursement policies.
Emerging markets such as Asia-Pacific and Latin America are anticipated to present significant expansion prospects in this space due to an increasing demand for neuroendoscopic products. Modern medical devices and an evolving healthcare infrastructure are revolutionizing patient care.
Recent advancements in this space include the launch of new neuroendoscopy devices with advanced features like augmented reality and real-time tracking, as well as innovations to existing ones.
The team behind market.us, marketresearch.biz, market.biz and more. Our purpose is to keep our customers ahead of the game with regard to the markets. They may fluctuate up or down, but we will help you to stay ahead of the curve in these market fluctuations. Our consistent growth and ability to deliver in-depth analyses and market insight has engaged genuine market players. They have faith in us to offer the data and information they require to make balanced and decisive marketing decisions.