Plasma Fractionation Market is Estimated to Showcase Significant Growth of USD 57.0 Bn in 2032 With a CAGR 7.3%

Page Contents
Market Overview
Plasma Fractionation is the process of separating plasma out of the blood and then processing it in order to make therapeutic products. These products are used for various medical conditions like bleeding disorders, immune disorders, neurological diseases, and other disorders. A rising number of diseases and the demand for plasma-derived therapies will drive significant growth in the plasma fractionation industry.
The global plasma fractionation market size is expected to be worth around USD 57.0 Bn by 2032 from USD 28.7 Bn in 2022, growing at a CAGR of 7.3% during the forecast period from 2022 to 2032.
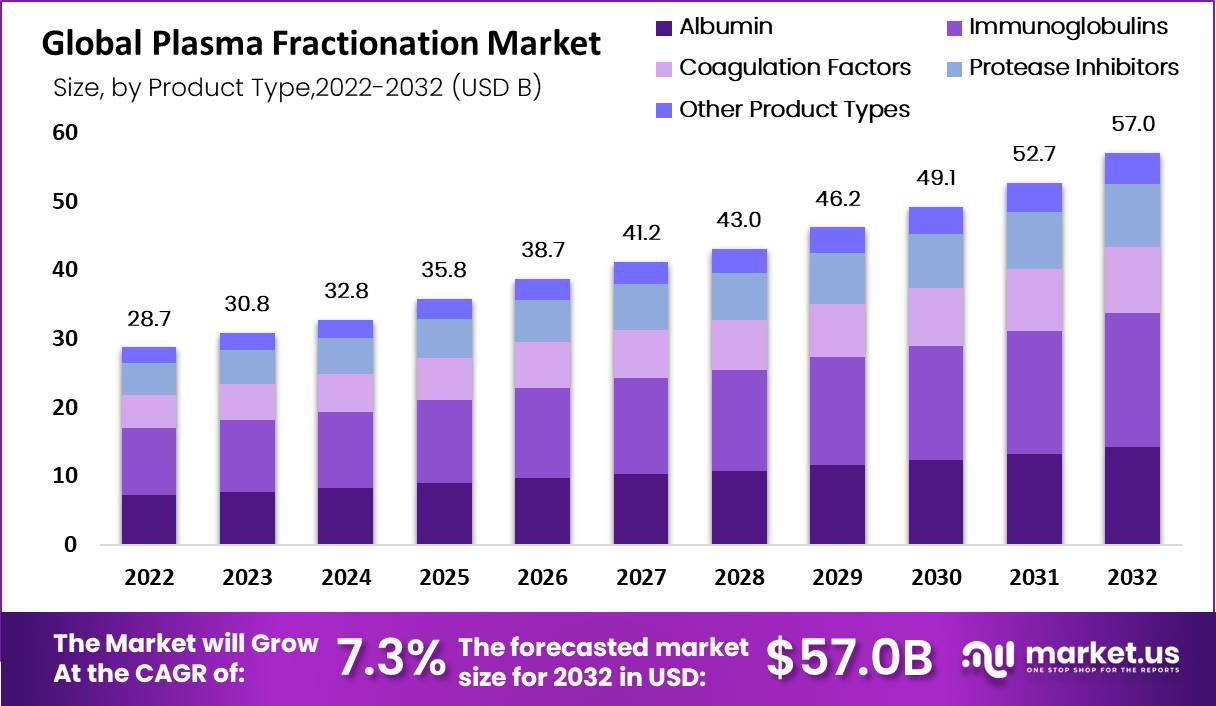
Key Takeaways
- Plasma fractionation market growth is anticipated to be 7.3% between 2023-2032.
- The key drivers for market growth are the rising prevalence of various diseases as well as the growing demand for plasma-derived therapies.
- The market is divided by product, geography, end users, and application.
- North America was the dominant market in 2020, owing to the presence of key players and high demand for plasma-derived therapies.
- CSL Behring (Grafols), Octapharma AG, and Biotest AG are the key players in this market. Kedrion S.p.A. is also a key player.
Drivers, trends, and challenges have an impact on market dynamics, which can impact businesses. Request for PDF sample report
Regional Snapshot
The plasma fractionation market can be divided into North America (Europe, Asia Pacific, Latin America, and Middle East & Africa). In 2020, North America was the dominant market due to the presence of key players and the high demand for plasma-derived therapeutics. Europe will see significant growth due both to the increasing demand for immunoglobulins products and the rising prevalence of chronic diseases.
Drivers
- Increased prevalence of many diseases
- Rising demand for plasma-derived therapies
- Plasma fractionation: Technological advances
- Plasma-derived therapies are becoming more popular
For many conditions, including neurologic, immunologic, or hematologic, immunoglobulins, are first-line therapies. The rate at which various immunological disorders have been diagnosed has increased over the last decade due to technological advances. The increased number of immunoglobulin-deficient patients will result in a greater clinical demand for immunoglobulins. This is due to genetic research being used to characterize and diagnose immunodeficiency. According to IG Living magazine, a publication for the immune globulin community, there are many IVIg on-and off-label indications. The use of off-label immunoglobulin indications has increased in recent years. They account for between 20-60% of clinical use.
Restraints
- High price for plasma-derived therapies
- Stringent regulations for plasma fractionation
Recombinant factors like AFSTYLA (CSL), VONVENDI(Takeda/Shire), and Kovaltrya (Bayer), have been approved recently. This market will offer companies lucrative opportunities. Two types of recombinant products are currently available: extended-half-life products or standard half-life. According to the Annual Global Survey of the World Federation of Hemophilia (WFH), VIII and IX are popular in developed countries. The use of recombinant elements has also increased in countries such as Brazil and India. This trend will likely limit the expansion of the plasma-derived coagulation factor, which will impact overall market growth.
Opportunities
- Increasing demand for albumin and immunoglobulin products
- Growing demand for plasma-derived therapies in emerging markets
- Expansion of product portfolios by key players
Challenges
- Limited plasma donor availability
- Plasma fractionation carries a high risk of infection
- Concerns about plasma donation and ethical issues
Plasma fractionation is one of the most tightly regulated areas in the pharmaceutical industry. There is a risk of blood-borne pathogens such as bacteria, viruses, and prions being transmitted and there are other diseases associated with transfusions. For this reason, regulators have focused on safety and quality in fractionated plasma products. To maintain safety and quality in plasma products, the WHO established guidelines. This market's strict regulations act as a barrier to new players and a restriction for local players looking to expand into the international market. Multinational companies face a variety of challenges when they invest in regional markets or local markets like China. China bans the import of plasma products (except albumin) since 1986. Also, it is difficult to obtain a license to establish a plasma collection center. These factors hinder the growth in China, the second-largest country for plasma fractionation.
Recent Developments
- CSL Behring launched HAEGARDA, a plasma-derived treatment for hereditary Angioedema (HAE) in March 2021.
- Octapharma AG announced that they had purchased 80% of Octagam from Bio Products Laboratory in January 2021. Octagam is a well-known immunoglobulin product.
- Grifols, a company that develops plasma-derived therapies, announced in November 2020 the launch of Xembify for primary immunodeficiency treatment.
Grow your profit margin with Market.us – Acquire this Report
Key Market Segments
Based on Product Type
- Albumin
- Immunoglobulins
- Coagulation Factors
- Protease Inhibitors
- Other Product Types
Based on Method
- Centrifugation
- Depth Filtration
- Chromatography
- Other Methods
Based on the Application
- Neurology
- Hematology
- Oncology
- Immunology
- Pulmonology
- Other Applications
Based on End-User
- Hospitals
- Clinical Research Laboratories
- Other End-Users
Market Key Players
- Bio Products Laboratory Biotest AG
- China Biologic
- Intas Pharmaceuticals Ltd.
- Products Holdings Inc.
- CSL Limited
- Takeda Pharmaceutical Company Limited
- Green Cross Corporation
- Grifols
- Other Key Players
Interested to Procure the Data? Inquire here at https://market.us/report/plasma-fractionation-market/#inquiry
Report Scope
| Report Attribute | Details |
| The market size value in 2022 | USD 28.7 Bn |
| Revenue forecast by 2032 | USD 57.0 Bn |
| Growth Rate | CAGR Of 7.3% |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, and the Rest of the World |
| Historical Years | 2017-2022 |
| Base Year | 2022 |
| Estimated Year | 2023 |
| Short-Term Projection Year | 2028 |
| Long-Term Projected Year | 2032 |
Frequently Asked Questions
Q: What is plasma fractionation?
A: Plasma fractionation is the process of separating plasma from blood and then processing it to produce various therapeutic products.
Q: What are the key drivers of the plasma fractionation market?
A: The key drivers of the plasma fractionation market include the increasing prevalence of various diseases, rising demand for plasma-derived therapies, technological advancements in plasma fractionation, and growing awareness about plasma-derived therapies.
Q: Which region dominated the plasma fractionation market in 2020?
A: North America dominated the plasma fractionation market in 2020 due to the presence of key players and the high demand for plasma-derived therapies.
Q: Who are the key players in the plasma fractionation market?
A: The key players in the plasma fractionation market include CSL Behring, Grifols, Octapharma AG, Biotest AG, Kedrion S.p.A., and Shanghai RAAS Blood Products Co. Ltd.
Q: What are the major challenges in the plasma fractionation market?
A: The major challenges in the plasma fractionation market include the limited availability of plasma donors and the high risk of infections associated with plasma fractionation.
The team behind market.us, marketresearch.biz, market.biz and more. Our purpose is to keep our customers ahead of the game with regard to the markets. They may fluctuate up or down, but we will help you to stay ahead of the curve in these market fluctuations. Our consistent growth and ability to deliver in-depth analyses and market insight has engaged genuine market players. They have faith in us to offer the data and information they require to make balanced and decisive marketing decisions.



